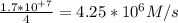Chemistry, 21.02.2020 18:30 naomirice24
The reaction between ethyl bromide (C₂H₅Br) and hydroxide ion in ethyl alcohol at 330 K, , is first order each in ethyl bromide and hydroxide ion. When [C₂H₅Br] is 0.0477 M and [OH⁻] is 0.100 M, the rate of disappearance of ethyl bromide is 1.7×10⁺⁷M/s.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
The reaction between ethyl bromide (C₂H₅Br) and hydroxide ion in ethyl alcohol at 330 K, , is first...
Questions

Mathematics, 04.11.2020 22:00

Mathematics, 04.11.2020 22:00


Mathematics, 04.11.2020 22:00

History, 04.11.2020 22:00


English, 04.11.2020 22:00

Mathematics, 04.11.2020 22:00

Social Studies, 04.11.2020 22:00

English, 04.11.2020 22:00


History, 04.11.2020 22:00

Mathematics, 04.11.2020 22:00


History, 04.11.2020 22:00





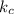 [A] where [A] is the concentration of the reagent
[A] where [A] is the concentration of the reagent![k_{c} \frac{[C_{2} H_{5} Br]}{2} \frac{[OH^{-} ]}{2}](/tpl/images/0519/3086/4c99a.png) =
= 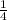 ×Kc[C₂H₅Br][OH⁻]
×Kc[C₂H₅Br][OH⁻]