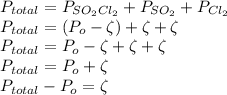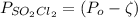As discussed in the text, the total system pressure can be used to monitor the progress of a chemical reaction. Consider the following reaction: SO2Cl2(g)→SO2(g)+Cl2(g). The reaction is initiated, and the following data are obtained:
Time (h) 0 3 6 9 12 15
PTotal (kPa) 11.07 14.79 17.26 18.90 19.99 20.71
Is the reaction first or second order with respect to SO2Cl2?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1


Chemistry, 23.06.2019 08:40
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1
You know the right answer?
As discussed in the text, the total system pressure can be used to monitor the progress of a chemica...
Questions



Business, 18.09.2019 01:40

Mathematics, 18.09.2019 01:40



Mathematics, 18.09.2019 01:40



Mathematics, 18.09.2019 01:40






Chemistry, 18.09.2019 01:40

Mathematics, 18.09.2019 01:40



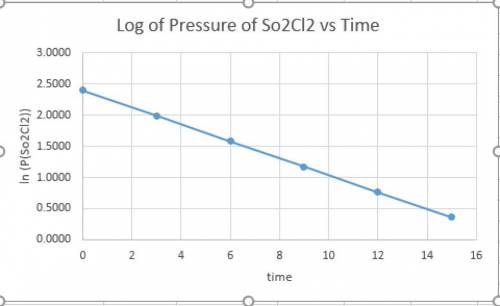
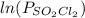 and time as given in the graph attached thus the reaction is first order wrt
and time as given in the graph attached thus the reaction is first order wrt 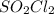 .
.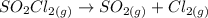
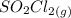 dissociates into exactly one mole of
dissociates into exactly one mole of 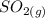 and
and 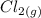 .
.