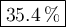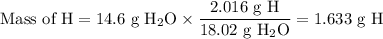Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 14:30
If energy was included in a chemical reaction, on which side of the equation would it be written for an endothermic reaction?
Answers: 1

Chemistry, 23.06.2019 17:10
Which substance produces hydroxide ions in solution? a. an arrhenius acid b. an arrhenius base c. a brønsted-lowry acid d. a brønsted-lowry base e. an amphoteric substance
Answers: 3
You know the right answer?
A 14.60g sample of an unknown compound, composed only of carbon, hydrogen, and oxygen, produced 28.6...
Questions

Business, 09.04.2021 14:50


Biology, 09.04.2021 14:50

Mathematics, 09.04.2021 14:50



Mathematics, 09.04.2021 14:50


Mathematics, 09.04.2021 14:50

English, 09.04.2021 14:50


Mathematics, 09.04.2021 14:50

Mathematics, 09.04.2021 14:50



Mathematics, 09.04.2021 14:50



Physics, 09.04.2021 14:50