
Chemistry, 21.02.2020 05:09 ilovepickles930
3.00 mol of an ideal gas with CV, m=3R/2 undergoes an expansion from an initial state described by T=310.K and P=6.00bar against a constant external pressure of zero bar until the final pressure is equal to one-fifth of its initial value. The state of the surroundings is T=298K, P=0.250bar. a. FInd entropy of surroundings.
b. Find total entropy

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2


Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
3.00 mol of an ideal gas with CV, m=3R/2 undergoes an expansion from an initial state described by T...
Questions




Computers and Technology, 05.11.2019 02:31
















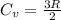
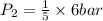 > \frac{1}{5} \times P_{1}[/tex]
> \frac{1}{5} \times P_{1}[/tex]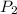 = 1.2 bar
= 1.2 bar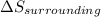 = 0
= 0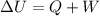

 = 0 this means that
= 0 this means that 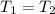
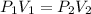


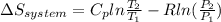
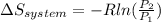
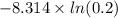


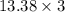
 > 0
> 0

