
Chemistry, 21.02.2020 04:48 algahimnada
A 6.19g sample of PCl5 is placed in an evacuated 2.00L flask and is completely vaporized at 252C. 1. Calculate the pressure in the flask if no chemical reaction were to occur. P=.6406 ATM 2.Actually at 252C the PCl5 is partially dissociated according to the following equation: PCl5<->PCl3+Cl2 (all gass states) The observed pressure is found to be 1.00 ATM. In view of this observation, calculate the partial pressure of PCl5 and PCl3 in the flask at 252C.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
A 6.19g sample of PCl5 is placed in an evacuated 2.00L flask and is completely vaporized at 252C. 1....
Questions


History, 28.08.2020 23:01



History, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01



Mathematics, 28.08.2020 23:01

Spanish, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01


Mathematics, 28.08.2020 23:01


Mathematics, 28.08.2020 23:01



Mathematics, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01

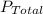 = P₁ + P₂ +...+Pₙ where P₁, P₂, Pₙ are the partial pressures of the constituent gases
= P₁ + P₂ +...+Pₙ where P₁, P₂, Pₙ are the partial pressures of the constituent gases


