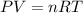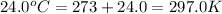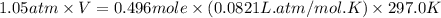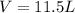Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

You know the right answer?
Ammonium carbonate decomposes upon heating according to the following balanced equation:
(NH4)...
(NH4)...
Questions

Biology, 26.11.2021 20:30


Biology, 26.11.2021 20:30

English, 26.11.2021 20:30





Arts, 26.11.2021 20:30

History, 26.11.2021 20:30



Physics, 26.11.2021 20:30

Chemistry, 26.11.2021 20:30



History, 26.11.2021 20:30

Mathematics, 26.11.2021 20:30

Social Studies, 26.11.2021 20:30

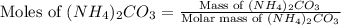
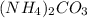 = 96.094 g/mol
= 96.094 g/mol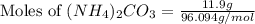
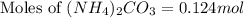
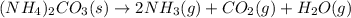
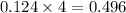 mole of gas
mole of gas