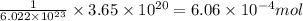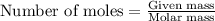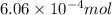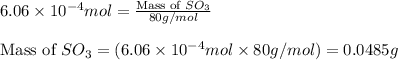Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1


Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

You know the right answer?
Sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain...
Questions

Mathematics, 27.01.2020 23:31

Business, 27.01.2020 23:31




Mathematics, 27.01.2020 23:31

Mathematics, 27.01.2020 23:31

Mathematics, 27.01.2020 23:31

Physics, 27.01.2020 23:31




History, 27.01.2020 23:31



Mathematics, 27.01.2020 23:31

Computers and Technology, 27.01.2020 23:31



Biology, 27.01.2020 23:31

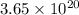
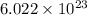 number of molecules are present in 1 mole of a compound
number of molecules are present in 1 mole of a compound