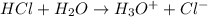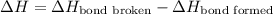Chemistry, 21.02.2020 02:31 chanelandme123
The O-H bond energy is 464 kJ/mol and the HI bond energy is 291 kJ/mol. Remembering that breaking any bond is an endothermic process and making any bond is exothermic, what is the net enthalpy change involved in the reaction between HI and H2O? Is this reaction endothermic or exothermic?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1


Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3
You know the right answer?
The O-H bond energy is 464 kJ/mol and the HI bond energy is 291 kJ/mol. Remembering that breaking an...
Questions


Social Studies, 23.09.2019 02:30

Mathematics, 23.09.2019 02:30

Social Studies, 23.09.2019 02:30

English, 23.09.2019 02:30

English, 23.09.2019 02:30

Mathematics, 23.09.2019 02:30



Mathematics, 23.09.2019 02:30

History, 23.09.2019 02:30

Biology, 23.09.2019 02:30

Business, 23.09.2019 02:30


English, 23.09.2019 02:30




Mathematics, 23.09.2019 02:30