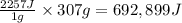Chemistry, 21.02.2020 02:28 akatian55721
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature. On average, an athlete loses approximately 307 g of sweat during an hour of exercise. How much energy is needed to evaporate the sweat that is produced? The heat of vaporization for water is 2257J/g. energy required:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature...
Questions

Mathematics, 30.11.2019 02:31




English, 30.11.2019 02:31



Mathematics, 30.11.2019 02:31

Social Studies, 30.11.2019 02:31








Mathematics, 30.11.2019 02:31


Computers and Technology, 30.11.2019 02:31

Mathematics, 30.11.2019 02:31