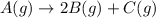Chemistry, 21.02.2020 01:15 ToonGamesToo
Consider the generic reaction: A (g) → 2 B (g) + C (g) . If a flask initially contains only A and the reaction proceeds to completion with a final pressure of 38.9 atm , what is the partial pressure of B in the container? (Assume constant volume and temperature.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Consider the generic reaction: A (g) → 2 B (g) + C (g) . If a flask initially contains only A and th...
Questions

Mathematics, 14.04.2020 22:30


Mathematics, 14.04.2020 22:30

Computers and Technology, 14.04.2020 22:30





Mathematics, 14.04.2020 22:30



English, 14.04.2020 22:30






Mathematics, 14.04.2020 22:31