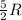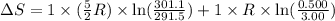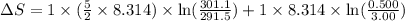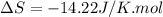Chemistry, 21.02.2020 01:05 AsiaDeas4078
During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 °C was compressed suddenly (and irrevers- ibly) to 0.500 L by driving in a piston. In the process, the tempera- ture of the gas increased to 28.1°C. Assume ideal behavior, what is the change in entropy of the gas?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

You know the right answer?
During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 °C was compressed s...
Questions




Chemistry, 21.11.2020 02:30

Arts, 21.11.2020 02:30

Mathematics, 21.11.2020 02:30





Mathematics, 21.11.2020 02:30

Mathematics, 21.11.2020 02:30


Mathematics, 21.11.2020 02:30

Arts, 21.11.2020 02:30

Biology, 21.11.2020 02:30

Mathematics, 21.11.2020 02:30

History, 21.11.2020 02:30


History, 21.11.2020 02:30

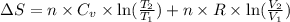
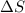 = change in molar entropy
= change in molar entropy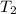 = final temperature =
= final temperature = 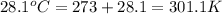
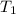 = initial temperature =
= initial temperature = 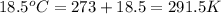
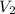 = final volume = 0.500 L
= final volume = 0.500 L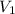 = initial volume = 3.00 L
= initial volume = 3.00 L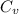 = heat capacity diatomic gas
= heat capacity diatomic gas 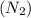 =
=