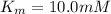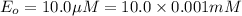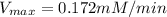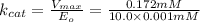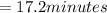Chemistry, 21.02.2020 01:03 adammouedden198
For an enzymatically catalyzed reaction in which the measured values for KM and Vmax are, respectively, 10.0 mM and 0.172 mM min-1, what is the value of the turnover number? The concentration of enzyme used in the reaction is 10.0 μM.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 23.06.2019 08:30
Explain how to convert from one unit to another in the metric system.
Answers: 3
You know the right answer?
For an enzymatically catalyzed reaction in which the measured values for KM and Vmax are, respective...
Questions

Mathematics, 11.08.2021 01:10



Biology, 11.08.2021 01:10


Mathematics, 11.08.2021 01:10




Social Studies, 11.08.2021 01:10





Mathematics, 11.08.2021 01:10



Engineering, 11.08.2021 01:10


![V = V_{max}\times \frac{[S]}{ (Km + [S])}](/tpl/images/0518/4358/1bc4a.png)
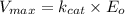
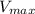 = max rate velocity
= max rate velocity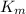 = Michaelis-Menten constant
= Michaelis-Menten constant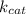 = catalytic rate constant
= catalytic rate constant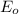 = initial enzyme concentration
= initial enzyme concentration