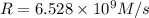Chemistry, 21.02.2020 00:00 ylianafghgfdsnm1479
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the following way:
OCl−+I−→OI−+Cl−.
This rapid reaction gives the following rate data:
[OCl−](M) [I]−(M) Rate (M/s)
1.5×10^−3 1.5×10^−3 1.36×10^−4
3.0×10^−3 1.5×10^−3 2.72×10^−4
1.5×10^−3 3.0×10^−3 2.72×10^−4
a. Write the rate law for this reaction.
b. Calculate the rate constant with proper units.
c. Calculate the rate when [OCl-]= 1.8×10^3 M and [I-]= 6.0×10^4 M .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the foll...
Questions

Computers and Technology, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01


Chemistry, 02.10.2020 14:01

History, 02.10.2020 14:01

Computers and Technology, 02.10.2020 14:01


Social Studies, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01



![R=k[OCl^-]^1\times [I^-]^1](/tpl/images/0518/3250/d583c.png)
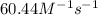 .
.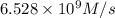 .
.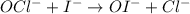
![R=k[OCl^-]^x\times [I^-]^y](/tpl/images/0518/3250/04fdb.png)
![[OCl^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/b77da.png) and
and ![[I^-]=1.5\times 10^{-3} M](/tpl/images/0518/3250/2f2e0.png) .
.![1.36\times 10^{-4} M/s=k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y](/tpl/images/0518/3250/e45d8.png) ..[1]
..[1]![[OCl^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/3317d.png) and
and ![2.72\times 10^{-4}M/s=k[3.0\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y](/tpl/images/0518/3250/dea0f.png) ..[2]
..[2]![[I^-]=3.0\times 10^{-3} M](/tpl/images/0518/3250/b24ad.png) .
.![2.72\times 10^{-4} M/s=k[1.5\times 10^{-3} M]^x\times [3.0\times 10^{-3} M]^y](/tpl/images/0518/3250/22116.png) ..[3]
..[3]![\frac{1.36\times 10^{-4}M/s}{2.72\times 10^{-4}M/s}=\frac{k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}{k[3.0\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}](/tpl/images/0518/3250/ebd3d.png)
![\frac{1.36\times 10^{-4} M/s}{2.72\times 10^{-4} M/s}=\frac{k[1.5\times 10^{-3} M]^x\times [1.5\times 10^{-3} M]^y}{k[1.5\times 10^{-3} M]^x\times [3.0\times 10^{-3} M]^y}](/tpl/images/0518/3250/11fc2.png)
![1.36\times 10^{-4} M/s=k[1.5\times 10^{-3} M]\times [1.5\times 10^{-3} M]](/tpl/images/0518/3250/f3ed7.png)
![k=\frac{1.36\times 10^{-4} M/s}{[1.5\times 10^{-3} M]\times [1.5\times 10^{-3} M]}=60.44 M^{-1}s^{-1}](/tpl/images/0518/3250/1ed07.png)
![[OCl^-]=1.8\times 10^{3} M](/tpl/images/0518/3250/b9482.png) and
and ![[I^-]=6.0\times 10^{4} M](/tpl/images/0518/3250/ceced.png) be R.
be R.![R=60.44 M^{-1}s^{-1}\times [1.8\times 10^{3} M]^1\times [6.0\times 10^{4} M]^1](/tpl/images/0518/3250/1384b.png)