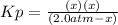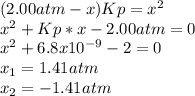Chemistry, 20.02.2020 23:05 transfergiecek8765
Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass. One of the compounds used to make Lexan is phosgene (COCl2), an extremely poisonous gas. Phosgene decomposes by the following reaction for which Kp = 6.8 ✕ 10-9 at 100°C. COCl2(g) equilibrium reaction arrow CO(g) + Cl2(g) If pure phosgene at an initial pressure of 2.0 atm decomposes, calculate the equilibrium pressures of all species.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass. One of the c...
Questions

English, 12.03.2021 19:20

English, 12.03.2021 19:20

Mathematics, 12.03.2021 19:20



Social Studies, 12.03.2021 19:20

Mathematics, 12.03.2021 19:20




Mathematics, 12.03.2021 19:20





Social Studies, 12.03.2021 19:20


Mathematics, 12.03.2021 19:20

Mathematics, 12.03.2021 19:20

Mathematics, 12.03.2021 19:20

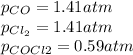
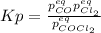
 , the law of mass action becomes:
, the law of mass action becomes: