
Chemistry, 20.02.2020 23:07 barbiegirllover
Iridium has only two naturally occurring isotopes. Ir-191 with arelative mass of 190.96058 and Ir-193 with a relative mass of192.96292. Compute the fractional abundance of Ir-191.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Iridium has only two naturally occurring isotopes. Ir-191 with arelative mass of 190.96058 and Ir-19...
Questions

Chemistry, 16.10.2020 18:01



Mathematics, 16.10.2020 18:01


Biology, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01


Social Studies, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Social Studies, 16.10.2020 18:01






Mathematics, 16.10.2020 18:01

Computers and Technology, 16.10.2020 18:01

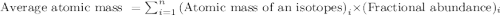 .....(1)
.....(1)![192.217=[(190.96058\times x)+(192.96292\times (1-x))]\\\\x=0.372](/tpl/images/0518/2031/79ae6.png)


