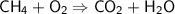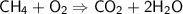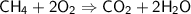Balance the following equation for the combustion of methane (3 pts):
CH4 + O2 --> C...

Chemistry, 20.02.2020 22:22 levicorey846
Balance the following equation for the combustion of methane (3 pts):
CH4 + O2 --> CO2 + H2O

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Questions

Mathematics, 17.05.2021 22:10


Mathematics, 17.05.2021 22:10

Mathematics, 17.05.2021 22:10


Mathematics, 17.05.2021 22:10

Mathematics, 17.05.2021 22:10


Biology, 17.05.2021 22:10

Mathematics, 17.05.2021 22:10





Mathematics, 17.05.2021 22:10

Physics, 17.05.2021 22:10


Social Studies, 17.05.2021 22:10

Mathematics, 17.05.2021 22:10

Mathematics, 17.05.2021 22:10

![\rule[225]{225}{2}](/tpl/images/0518/0259/85973.png)