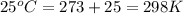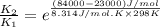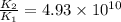Chemistry, 20.02.2020 22:23 electronia
The activation energy for a reaction is 84.0kJ/mol. Addition of a catalyst lowers the activation energy by 23.0 kJ/mol, while leaving the A-factor unchanged. By what factor ( uncatalyzed catalyzed k k ) does the rate increase at 25 °C?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3


Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
The activation energy for a reaction is 84.0kJ/mol. Addition of a catalyst lowers the activation ene...
Questions



Mathematics, 14.04.2021 18:30


Mathematics, 14.04.2021 18:30


Biology, 14.04.2021 18:30

Mathematics, 14.04.2021 18:30



Mathematics, 14.04.2021 18:30

Physics, 14.04.2021 18:30





Computers and Technology, 14.04.2021 18:30



Chemistry, 14.04.2021 18:30

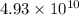
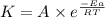
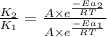
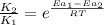
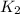 = rate of reaction with catalyst
= rate of reaction with catalyst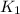 = rate of reaction without catalyst
= rate of reaction without catalyst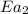 = activation energy with catalyst = 23.0 kJ/mol = 23000 J/mol
= activation energy with catalyst = 23.0 kJ/mol = 23000 J/mol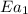 = activation energy without catalyst = 84.0 kJ/mol = 84000 J/mol
= activation energy without catalyst = 84.0 kJ/mol = 84000 J/mol