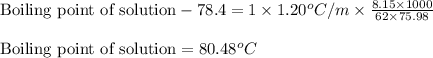Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1


Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Calculate the freezing point and boiling point of a solution containing 8.15 g of ethylene glycol (C...
Questions

History, 06.11.2020 03:10





Mathematics, 06.11.2020 03:10


Advanced Placement (AP), 06.11.2020 03:10


Mathematics, 06.11.2020 03:10

Mathematics, 06.11.2020 03:10

History, 06.11.2020 03:10


Physics, 06.11.2020 03:10

Mathematics, 06.11.2020 03:10

Arts, 06.11.2020 03:10

Mathematics, 06.11.2020 03:10

Mathematics, 06.11.2020 03:10

Mathematics, 06.11.2020 03:10

Mathematics, 06.11.2020 03:10

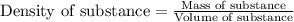
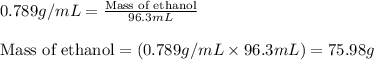
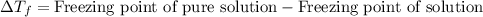
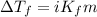
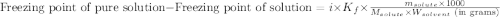
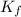 = molal freezing point elevation constant = 1.99°C/m
= molal freezing point elevation constant = 1.99°C/m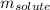 = Given mass of solute (ethylene glycol) = 8.15 g
= Given mass of solute (ethylene glycol) = 8.15 g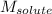 = Molar mass of solute (ethylene glycol) = 62 g/mol
= Molar mass of solute (ethylene glycol) = 62 g/mol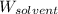 = Mass of solvent (ethanol) = 75.98 g
= Mass of solvent (ethanol) = 75.98 g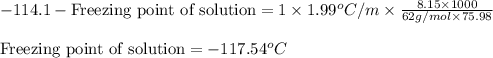
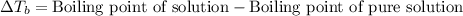
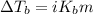
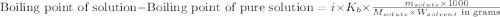
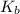 = molal boiling point elevation constant = 1.20°C/m.g
= molal boiling point elevation constant = 1.20°C/m.g