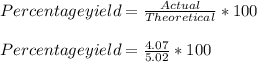Chemistry, 20.02.2020 19:20 laniflower737
A 5.95-g sample of AgNO3 is reacted with BaCl2 according to the equation to give 4.07 g of AgCl. What is the percent yield of AgCl?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1



You know the right answer?
A 5.95-g sample of AgNO3 is reacted with BaCl2 according to the equation to give 4.07 g of AgCl. Wha...
Questions



History, 10.12.2020 09:00

Mathematics, 10.12.2020 09:00

English, 10.12.2020 09:00

History, 10.12.2020 09:00

Mathematics, 10.12.2020 09:00


Mathematics, 10.12.2020 09:00

Mathematics, 10.12.2020 09:00

Mathematics, 10.12.2020 09:00






Physics, 10.12.2020 09:00



Mathematics, 10.12.2020 09:00

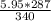 = 5.02 g of AgCl.
= 5.02 g of AgCl.