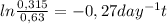Chemistry, 20.02.2020 16:50 meandmycousinmagic
A city's water supply is contaminated with a toxin at a concentration of 0.63 mg/L. Fortunately, this toxin decomposes to a safe mixture of products by first-order kinetics with a rate constant of 0.27 day–1. How long will it take for half of the toxin to decompose?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

You know the right answer?
A city's water supply is contaminated with a toxin at a concentration of 0.63 mg/L. Fortunately, thi...
Questions



Mathematics, 04.03.2020 05:01


Mathematics, 04.03.2020 05:16

Social Studies, 04.03.2020 05:16

Mathematics, 04.03.2020 05:16

Mathematics, 04.03.2020 05:17

Mathematics, 04.03.2020 05:18

Mathematics, 04.03.2020 05:18

Chemistry, 04.03.2020 05:18



Mathematics, 04.03.2020 05:18

Mathematics, 04.03.2020 05:18



English, 04.03.2020 05:20

History, 04.03.2020 05:20

![ln\frac{[A]_t}{[A]_0} = -kt](/tpl/images/0517/4629/57ff6.png)