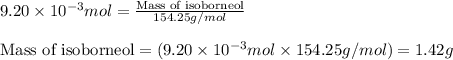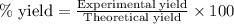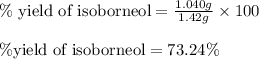Chemistry, 20.02.2020 08:02 tahjaybenloss16
Consider the sodium borohydride reduction of camphor to isoborneol. A reaction was performed in which 1.400 1.400 g of camphor was reduced by an excess of sodium borohydride to make 1.040 1.040 g of isoborneol. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
Consider the sodium borohydride reduction of camphor to isoborneol. A reaction was performed in whic...
Questions

Mathematics, 07.07.2019 15:00


Mathematics, 07.07.2019 15:00






Mathematics, 07.07.2019 15:00


Mathematics, 07.07.2019 15:00

Physics, 07.07.2019 15:00


History, 07.07.2019 15:00

Computers and Technology, 07.07.2019 15:00


History, 07.07.2019 15:00

History, 07.07.2019 15:00


English, 07.07.2019 15:00

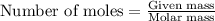 .....(1)
.....(1)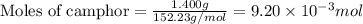
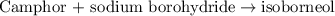
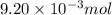 of camphor will produce =
of camphor will produce = 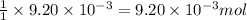 of isoborneol
of isoborneol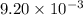 moles
moles