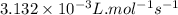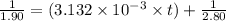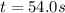Chemistry, 20.02.2020 09:40 oofoofoof1
The decomposition of NO2(g) occurs by the following bimolecular elementary reaction. 2NO2(g) → 2NO(g) + O2(g) The rate constant at 273 K is 2.3 x 10-12 L mol-1 s-1, and the activation energy is 111 kJ/mol. How long will it take (in s) for the concentration of NO2(g) to decrease from an initial partial pressure of 2.80 atm to 1.90 atm at 479 K? Assume ideal gas behavior.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
The decomposition of NO2(g) occurs by the following bimolecular elementary reaction. 2NO2(g) → 2NO(g...
Questions

Mathematics, 19.09.2021 22:00


Physics, 19.09.2021 22:00

Arts, 19.09.2021 22:00

Health, 19.09.2021 22:00


Mathematics, 19.09.2021 22:00

Mathematics, 19.09.2021 22:00

History, 19.09.2021 22:00


Mathematics, 19.09.2021 22:00

Mathematics, 19.09.2021 22:00

Mathematics, 19.09.2021 22:00

Mathematics, 19.09.2021 22:00

Mathematics, 19.09.2021 22:00


Mathematics, 19.09.2021 22:00



Mathematics, 19.09.2021 22:00

![\ln(\frac{K_{479K}}{K_{273K}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0517/3905/b7df8.png)
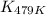 = equilibrium constant at 479 K = ?
= equilibrium constant at 479 K = ?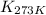 = equilibrium constant at 273 K =
= equilibrium constant at 273 K = 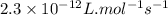
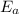 = Activation energy = 111 kJ/mol = 111000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 111 kJ/mol = 111000 J/mol (Conversion factor: 1 kJ = 1000 J)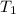 = initial temperature = 273 K
= initial temperature = 273 K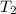 = final temperature = 479 K
= final temperature = 479 K![\ln(\frac{K_{479K}}{2.3\times 10^{-12}})=\frac{111000J}{8.314J/mol.K}[\frac{1}{273}-\frac{1}{479}]\\\\K_{479K}=3.132\times 10^{-3}L.mol^{-1}s^{-1}](/tpl/images/0517/3905/9840b.png)
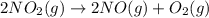
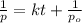
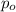 = initial partial pressure = 2.80 atm
= initial partial pressure = 2.80 atm