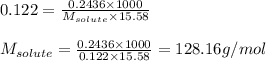Chemistry, 20.02.2020 03:33 cheerleader791
A. 0.2436 sample of an unknown substance was dissolved in 20.0mL of cyclohexane. The density of cyclohexane is 0.779 g/mL. The freezing point depression was 2.50 oC and the Kf value for cyclohecane is 20.5oC/m. Calculate the molality of the above solution and the molar mass of unknown.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
A. 0.2436 sample of an unknown substance was dissolved in 20.0mL of cyclohexane. The density of cycl...
Questions


English, 04.09.2019 03:20

Arts, 04.09.2019 03:20


Mathematics, 04.09.2019 03:20

Mathematics, 04.09.2019 03:20



History, 04.09.2019 03:20










Mathematics, 04.09.2019 03:30

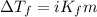
 = depression in freezing point = 2.50°C
= depression in freezing point = 2.50°C = molal freezing point elevation constant = 20.5°C/m
= molal freezing point elevation constant = 20.5°C/m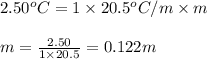
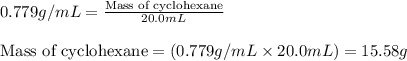
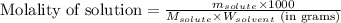
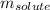 = Given mass of solute = 0.2436 g
= Given mass of solute = 0.2436 g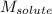 = Molar mass of solute = ? g/mol
= Molar mass of solute = ? g/mol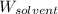 = Mass of solvent (cyclohexane) = 15.58 g
= Mass of solvent (cyclohexane) = 15.58 g