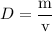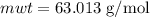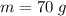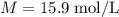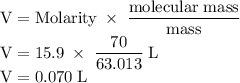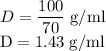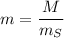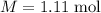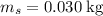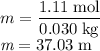Chemistry, 20.02.2020 03:38 anabelleacunamu
The concentration of commercially available concentrated nitric acid is 70.0 percent by mass, or 15.9 M. Calculate the density and molality of the solution.\

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
The concentration of commercially available concentrated nitric acid is 70.0 percent by mass, or 15....
Questions

Biology, 29.02.2020 01:27






Computers and Technology, 29.02.2020 01:27



Mathematics, 29.02.2020 01:27

Computers and Technology, 29.02.2020 01:27


Mathematics, 29.02.2020 01:27






History, 29.02.2020 01:27