

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
I2 => 2 I I + H2 => H2I H2I + I => 2 HI What is the molecularity of step 2? A. unimolecular...
Questions


Mathematics, 29.05.2020 14:57

English, 29.05.2020 14:57

History, 29.05.2020 14:57




Mathematics, 29.05.2020 14:57


Mathematics, 29.05.2020 14:57

Advanced Placement (AP), 29.05.2020 14:57






Mathematics, 29.05.2020 14:57



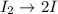
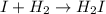
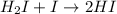
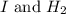 .
.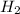 molecule.
molecule.

