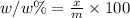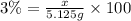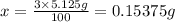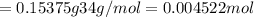Chemistry, 20.02.2020 01:43 helpmeplz33
If you are using 3.00% (mass/mass) hydrogen peroxide solution and you determine that the mass of solution required to reach the equivalence point is 5.125 g, how many moles of hydrogen peroxide molecules are present?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
If you are using 3.00% (mass/mass) hydrogen peroxide solution and you determine that the mass of sol...
Questions

Mathematics, 18.10.2020 15:01

Chemistry, 18.10.2020 15:01


English, 18.10.2020 15:01


Chemistry, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Chemistry, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Chemistry, 18.10.2020 15:01

English, 18.10.2020 15:01



English, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Biology, 18.10.2020 15:01

Arts, 18.10.2020 15:01