
Chemistry, 20.02.2020 01:10 gabrielolivas59
The percentage deprotonation of a 0.115 M solution of benzoic acid (a weak, monoprotic acid) is 2.1%. What is the pH of the solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3


Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
The percentage deprotonation of a 0.115 M solution of benzoic acid (a weak, monoprotic acid) is 2.1%...
Questions

Mathematics, 13.05.2020 03:57


Mathematics, 13.05.2020 03:57

Arts, 13.05.2020 03:57

History, 13.05.2020 03:57








Chemistry, 13.05.2020 04:57


Mathematics, 13.05.2020 04:57

Mathematics, 13.05.2020 04:57




 =
= 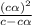 ( HA ---> H⁺A⁻ )
( HA ---> H⁺A⁻ )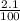 = 0.021
= 0.021 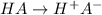
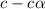
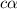
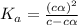
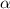 =
= 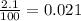
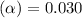
![[H^+]=c\times \alpha](/tpl/images/0516/4496/4fc41.png)
![[H^+]=0.115\times 0.021=0.0024](/tpl/images/0516/4496/7e639.png)
![pH=-log[H^+]](/tpl/images/0516/4496/15713.png)
![pH=-log[0.0024]=2.6](/tpl/images/0516/4496/aa86c.png)


