
Chemistry, 19.02.2020 22:59 Picklehead1166
Sodium metal (atomic weight 22.99 g/cm^3) adopts a body-centered cubic structure with a density of 0.97 g/cm^3. (a) Use this information and Avogrado's number (Na=6.022x10^23) to estimate the atomic radius of sodium. (b) If it didn't react so vigorously, sodium could float on water. Use the answer from part (a) to estimate the density of Na if its structure were that of a cubic close-packed metal. Would it still float on the water?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Sodium metal (atomic weight 22.99 g/cm^3) adopts a body-centered cubic structure with a density of 0...
Questions

English, 24.11.2020 17:30


Physics, 24.11.2020 17:30

Mathematics, 24.11.2020 17:30

World Languages, 24.11.2020 17:30

Social Studies, 24.11.2020 17:30

English, 24.11.2020 17:30



Physics, 24.11.2020 17:30

Health, 24.11.2020 17:30

Mathematics, 24.11.2020 17:30

History, 24.11.2020 17:30



Biology, 24.11.2020 17:30


Computers and Technology, 24.11.2020 17:30

Mathematics, 24.11.2020 17:30

Biology, 24.11.2020 17:30

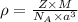
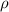 = density =
= density = 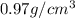
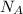 = Avogadro's number =
= Avogadro's number = 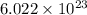
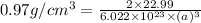
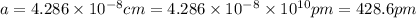
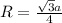
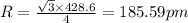
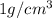
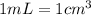 )
)

