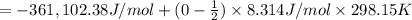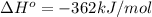A sample of K(s) of mass 2.720 g undergoes combustion in a constant volume calorimeter at 298.15 K. The calorimeter constant is 1849 J K−1, and the measured temperature rise in the inner water bath containing 1439 g of water is 1.60 K.
Part A
Calculate ΔU∘f for K2O.
Express your answer to three significant figures and include the appropriate units.
Part B
Calculate ΔH∘f for K2O.
Express your answer to three significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1
You know the right answer?
A sample of K(s) of mass 2.720 g undergoes combustion in a constant volume calorimeter at 298.15 K....
Questions


Mathematics, 14.12.2021 05:50

Mathematics, 14.12.2021 05:50

SAT, 14.12.2021 05:50

SAT, 14.12.2021 05:50

History, 14.12.2021 05:50

Mathematics, 14.12.2021 05:50

Mathematics, 14.12.2021 05:50


Mathematics, 14.12.2021 05:50


History, 14.12.2021 05:50


Mathematics, 14.12.2021 05:50

History, 14.12.2021 05:50

Mathematics, 14.12.2021 05:50

Mathematics, 14.12.2021 05:50

Mathematics, 14.12.2021 05:50

Mathematics, 14.12.2021 05:50

Business, 14.12.2021 05:50

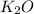 is -361 kJ/mol.
is -361 kJ/mol.![q=[q_1+q_2]](/tpl/images/0516/2162/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0516/2162/1d50b.png)
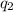 = heat absorbed by the water
= heat absorbed by the water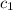 = specific heat of calorimeter =
= specific heat of calorimeter = 
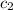 = specific heat of water =
= specific heat of water = 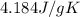
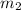 = mass of water = 1439 g
= mass of water = 1439 g = change in temperature =
= change in temperature = 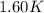
![q=[(1849 J/K \times 1.60 K)+(1439 g \times 4.184J/gK\times 1.60 K)]](/tpl/images/0516/2162/36fc3.png)

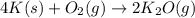
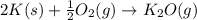
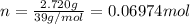
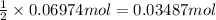 of
of 
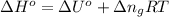
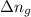 = moles of gases on RHS - moles of gasses on LHS
= moles of gases on RHS - moles of gasses on LHS