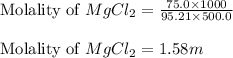Chemistry, 19.02.2020 21:00 mckenziet8930
Calculate the molality of 75.0 grams of MgCl2 (molar mass=95.21 g/mol) dissolved in 500.0 g of solvent.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
Calculate the molality of 75.0 grams of MgCl2 (molar mass=95.21 g/mol) dissolved in 500.0 g of solve...
Questions






Arts, 22.10.2020 20:01








Mathematics, 22.10.2020 20:01

History, 22.10.2020 20:01



Chemistry, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

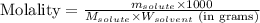
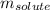 = Given mass of solute (magnesium chloride) = 75.0
= Given mass of solute (magnesium chloride) = 75.0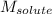 = Molar mass of solute (magnesium chloride) = 95.21 g/mol
= Molar mass of solute (magnesium chloride) = 95.21 g/mol 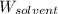 = Mass of solvent = 500.0 g
= Mass of solvent = 500.0 g