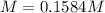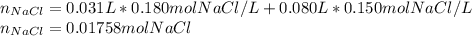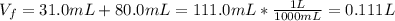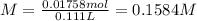Chemistry, 19.02.2020 20:04 taridunkley724
Calculate the molarity of the solution produced by mixing 31.0 mL of 0.180 M NaCl and 80.0 mL of 0.150 M NaCl. (Assume that the volumes are additive.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1


Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Calculate the molarity of the solution produced by mixing 31.0 mL of 0.180 M NaCl and 80.0 mL of 0.1...
Questions


Physics, 24.05.2021 08:40


Mathematics, 24.05.2021 08:40


Mathematics, 24.05.2021 08:40




Health, 24.05.2021 08:40



Biology, 24.05.2021 08:40

Mathematics, 24.05.2021 08:40

History, 24.05.2021 08:40

English, 24.05.2021 08:40

Biology, 24.05.2021 08:40

Mathematics, 24.05.2021 08:40


Mathematics, 24.05.2021 08:40