Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1


Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

You know the right answer?
Acetylsalicylic acid (aspirin), HC9H7O4, is the most widely used pain reliever and fever reducer. Fi...
Questions


Mathematics, 06.05.2020 05:44


Mathematics, 06.05.2020 05:44





Mathematics, 06.05.2020 05:44

Mathematics, 06.05.2020 05:44

Mathematics, 06.05.2020 05:44

Mathematics, 06.05.2020 05:44

Chemistry, 06.05.2020 05:44



Mathematics, 06.05.2020 05:44

History, 06.05.2020 05:44

Biology, 06.05.2020 05:44


History, 06.05.2020 05:44

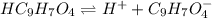
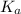 for above equation follows:
for above equation follows:![K_a=\frac{[C_9H_7O_4^-][H^+]}{[HC_9H_7O_4]}](/tpl/images/0515/8851/cb0ef.png)
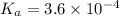
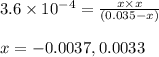
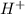 = x = 0.0033 M
= x = 0.0033 M![pH=-\log[H^+]](/tpl/images/0515/8851/cf945.png)