
Chemistry, 19.02.2020 05:52 animerocks07
Determining reaction order : Rate Laws(Chemistry)
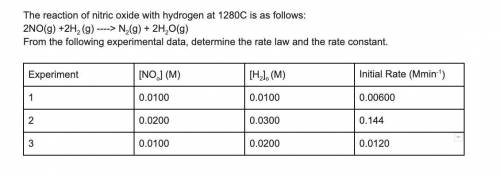
The reaction of nitric oxide with hydrogen at 1280C is as follows:
2NO(g) +2H2 (g) -> N2(g) + 2H2O(g)
From the following experimental data, determine the rate law and the rate constant.
30 POINTS!

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
Determining reaction order : Rate Laws(Chemistry)
The reaction of nitric oxide with hydrogen...
The reaction of nitric oxide with hydrogen...
Questions


Social Studies, 09.01.2022 01:00


Mathematics, 09.01.2022 01:00

Mathematics, 09.01.2022 01:00

English, 09.01.2022 01:00


Social Studies, 09.01.2022 01:00



Mathematics, 09.01.2022 01:00

Biology, 09.01.2022 01:00

History, 09.01.2022 01:00

Mathematics, 09.01.2022 01:00

Physics, 09.01.2022 01:00


Mathematics, 09.01.2022 01:00

History, 09.01.2022 01:00

Mathematics, 09.01.2022 01:00

English, 09.01.2022 01:00

![R=k[NO]3[H_2]^1](/tpl/images/0515/4935/da8f4.png)
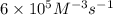
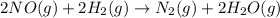
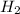 in rate law be x and y .
in rate law be x and y .![R=k[NO]^[H_2]^y](/tpl/images/0515/4935/cf38a.png)
![[NO]=0.0100 M, [H_2]=0.0100 M](/tpl/images/0515/4935/aa668.png)
![0.00600 M/s=k[0.0100 M]^x[0.0100 M]^y](/tpl/images/0515/4935/a6007.png) ..[1]
..[1]![[NO]=0.0200 M, [H_2]=0.0300 M](/tpl/images/0515/4935/ba703.png)
![0.144 M/s=k[0.0200 M]^x[0.0300 M]^y](/tpl/images/0515/4935/0042d.png) ..[2]
..[2]![[NO]=0.0100 M, [H_2]=0.0200 M](/tpl/images/0515/4935/ba63c.png)
![0.0120 M/s=k[0.0100 M]^x[0.0200 M]^y](/tpl/images/0515/4935/9f0d9.png) ..[3]
..[3]![\frac{0.00600 M/s}{0.0120 M/s}=\frac{k[0.0100 M]^x\times [0.0100 M]^y}{k[0.0100 M]^x\times [0.0200 M]^y}](/tpl/images/0515/4935/1b196.png)
![\frac{0.00600 M/s}{0.144 M/s}=\frac{k[0.0100 M]^x\times [0.0100 M]^1}{k[0.0200 M]^x\times [0.0300 M]^1}](/tpl/images/0515/4935/e9761.png)
![0.00600 M/s=k[0.0100 M]^3[0.0100 M]^1](/tpl/images/0515/4935/2cbdd.png) ..[1]
..[1]![k=\frac{0.00600 M/s}{[0.0100 M]^3[0.0100 M]^1}=6\times 10^5 M^{-3}s^{-1}](/tpl/images/0515/4935/88d8d.png)


