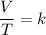Chemistry, 19.02.2020 04:54 tabisulaprinces2084
In the mathematical equation showing that volume (V) divided by temperature (T) remains equal to a constant (k), volume and temperature are proportional

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

You know the right answer?
In the mathematical equation showing that volume (V) divided by temperature (T) remains equal to a c...
Questions



Medicine, 12.12.2020 16:00

SAT, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00

Social Studies, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00


Social Studies, 12.12.2020 16:00

English, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

English, 12.12.2020 16:00

Biology, 12.12.2020 16:00


Computers and Technology, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00