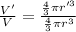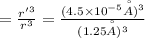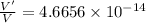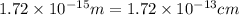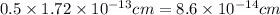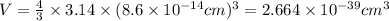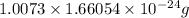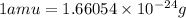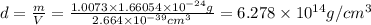An atom has a diameter of 2.50 Å and the nucleus of that atom has a diameter of 9.00×10−5 Å . Determine the fraction of the volume of the atom that is taken up by the nucleus. Assume the atom and the nucleus are a sphere.
fraction of atomic volume: ?
Calculate the density of a proton, given that the mass of a proton is 1.0073 amu and the diameter of a proton is 1.72×10−15 m.
density: ? g/cm^3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
An atom has a diameter of 2.50 Å and the nucleus of that atom has a diameter of 9.00×10−5 Å . Determ...
Questions




Biology, 01.04.2021 08:30

Advanced Placement (AP), 01.04.2021 08:30

Biology, 01.04.2021 08:30

Mathematics, 01.04.2021 08:30





Social Studies, 01.04.2021 08:30


History, 01.04.2021 08:30

History, 01.04.2021 08:30

Biology, 01.04.2021 08:30

Chemistry, 01.04.2021 08:40

English, 01.04.2021 08:40


Social Studies, 01.04.2021 08:40

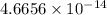 .
.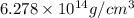 .
.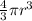
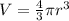 ..[1]
..[1]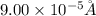
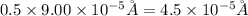
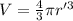 ..[2]
..[2]