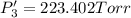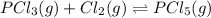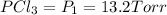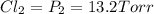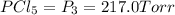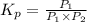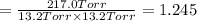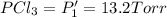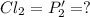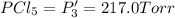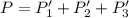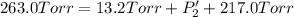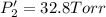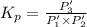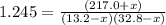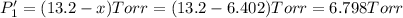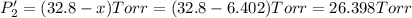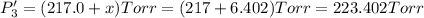Chemistry, 19.02.2020 03:25 erikabermudez55
An equilibrium mixture of PCl5(g), PCl3(g), and Cl2(g) has partial pressures of 217.0 Torr, 13.2 Torr, and 13.2 Torr, respectively. A quantity of Cl2(g) is injected into the mixture, and the total pressure jumps to 263.0 Torr (at the moment of mixing). The system then re-equilibrates. The appropriate chemical equation is:
PCl3(g) + Cl2(g) ---> PCl5(g)
Calculate the new partial pressures after equilibrium is reestablished. [in torr]
PPCl3
PPCl2
PPCl5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1


Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
An equilibrium mixture of PCl5(g), PCl3(g), and Cl2(g) has partial pressures of 217.0 Torr, 13.2 Tor...
Questions


Mathematics, 27.09.2021 09:00

Mathematics, 27.09.2021 09:00

English, 27.09.2021 09:00



Mathematics, 27.09.2021 09:00

Mathematics, 27.09.2021 09:00

Mathematics, 27.09.2021 09:00



History, 27.09.2021 09:00



Biology, 27.09.2021 09:00


Mathematics, 27.09.2021 09:00



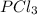 :
: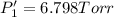
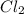 :
: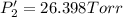
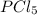 :
: