
Chemistry, 19.02.2020 02:45 blayneaafedt
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, 50 Cr , 52 Cr , 53 Cr , and 54 Cr . The 52 Cr isotope has a natural abundance of 83.79 % and an atomic mass of 51.9405 u. The 54 Cr isotope has a natural abundance of 2.37 % and an atomic mass of 53.9389 u. The natural abundances of the 50 Cr and 53 Cr isotopes exist in a ratio of 0.4579 : 1 , and the 50 Cr isotope has an atomic mass of 49.9460 u. Determine the atomic mass of the 53 Cr isotope. atomic mass of 53 Cr:.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, 50 Cr , 52 Cr , 53 Cr , and...
Questions


Mathematics, 24.10.2020 02:00


Mathematics, 24.10.2020 02:00



English, 24.10.2020 02:00


Chemistry, 24.10.2020 02:00


Biology, 24.10.2020 02:00


Mathematics, 24.10.2020 02:00

English, 24.10.2020 02:00

English, 24.10.2020 02:00

Mathematics, 24.10.2020 02:00

Biology, 24.10.2020 02:00


History, 24.10.2020 02:00

Mathematics, 24.10.2020 02:00

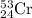 isotope is 52.8367 amu
isotope is 52.8367 amu![_{24}^{50}\text{Cr}\text{ and }_{24}^{53}\textrm{Cr}\text{ isotopes}=[100-(83.79+2.37)]=13.84\%](/tpl/images/0515/1858/e24f2.png)
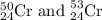 isotopes = 0.4579 : 1
isotopes = 0.4579 : 1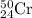 isotope =
isotope = 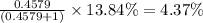
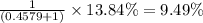
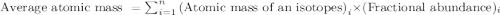 .....(1)
.....(1)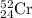 isotope:
isotope: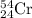 isotope:
isotope:![51.9961=[(49.9460\times 0.0437)+(51.9405\times 0.8379)+(x\times 0.0949)+(53.9389\times 0.0237)]\\\\x=52.8367amu](/tpl/images/0515/1858/822e6.png)



