
Consider the following system at equilibrium: 2A(aq)+2B(aq)⇌5C(aq) Classify each of the following actions by whether it causes a leftward shift, a rightward shift, or no shift in the direction of the net reaction. a. increase (b)
b. increase(a)
c. increase(c)
d. decrease(a)
e. decrease(b)
f. decrease(c)
g. double(a) and reduce (b) to one half
h. double both (b) and (c)

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

You know the right answer?
Consider the following system at equilibrium: 2A(aq)+2B(aq)⇌5C(aq) Classify each of the following ac...
Questions

History, 13.11.2020 19:50

History, 13.11.2020 19:50




History, 13.11.2020 19:50

Arts, 13.11.2020 19:50


English, 13.11.2020 19:50

Biology, 13.11.2020 20:00

Mathematics, 13.11.2020 20:00




Biology, 13.11.2020 20:00

Physics, 13.11.2020 20:00


Mathematics, 13.11.2020 20:00

Biology, 13.11.2020 20:00

Mathematics, 13.11.2020 20:00

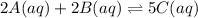
![K_{c}=\frac{[C(aq)]^5}{[A(aq)]^2\cdot [B(aq)]^2}](/tpl/images/0515/1184/e02e8.png)
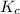 is the same, meaning that an increase in the concentration of the species B must cause a rightward shift to increase the concentration of the species C, such that the ratio expressed by the equilibrium constant remains unchanged.
is the same, meaning that an increase in the concentration of the species B must cause a rightward shift to increase the concentration of the species C, such that the ratio expressed by the equilibrium constant remains unchanged.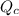 to compare with the equilibrium constant
to compare with the equilibrium constant ![Q{c}=\frac{[C(aq)]^5}{[A(aq)]^2\cdot [B(aq)]^2}](/tpl/images/0515/1184/564e9.png)



