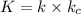Chemistry, 19.02.2020 01:25 NetherisIsTheQueen
The following mechanism has been proposed for the gas phase reaction of nitrogen monoxide with bromine.
step 1 fast: NO Br2 NOBr2
step 2 slow: NOBr2 NO 2 NOBr
(1) What is the equation for the overall reaction
(2) Enter the formula of any species that acts as a reaction intermediate?
(3) Complete the rate law for the overall reaction that is consistent with this mechanism.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
The following mechanism has been proposed for the gas phase reaction of nitrogen monoxide with bromi...
Questions

Mathematics, 24.03.2020 21:43










Computers and Technology, 24.03.2020 21:43



Mathematics, 24.03.2020 21:43

Mathematics, 24.03.2020 21:43

English, 24.03.2020 21:43

Mathematics, 24.03.2020 21:43

History, 24.03.2020 21:43

Mathematics, 24.03.2020 21:43

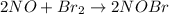
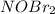 .
.![R=K[NO]^2[Br]](/tpl/images/0515/0512/945c3.png)
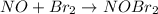 ..[1]
..[1]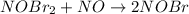 ...[2]
...[2]![R=k[NOBr_2][NO]](/tpl/images/0515/0512/7dc1f.png) ..[3]
..[3]![K_c=\frac{[NOBr_2]}{[NO][Br_2]}](/tpl/images/0515/0512/8d77b.png)
![[NOBr_2]=K_c\times [NO][Br_2]](/tpl/images/0515/0512/df1dc.png)
![[NOBr_2]](/tpl/images/0515/0512/86582.png) rate expression [3]:
rate expression [3]:![R=k\times k_c[NO][NO][NO]=K[NO]^2[Br]](/tpl/images/0515/0512/f99df.png)