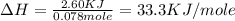Chemistry, 19.02.2020 00:21 carlyfaith3375
When 60 mL of 1.30 mol/L AgNO3(aq) and 60 mL of 1.30 mol/L HCl(aq) are mixed in a simple calorimeter, the temperature rises by 5.18°C. The molar enthalpy of reaction of HCl(aq) is ab. C kJ/mol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1
You know the right answer?
When 60 mL of 1.30 mol/L AgNO3(aq) and 60 mL of 1.30 mol/L HCl(aq) are mixed in a simple calorimeter...
Questions


Physics, 29.04.2021 23:50



Mathematics, 29.04.2021 23:50

Mathematics, 29.04.2021 23:50

Mathematics, 29.04.2021 23:50


Mathematics, 29.04.2021 23:50




Physics, 29.04.2021 23:50

History, 29.04.2021 23:50


English, 29.04.2021 23:50



Social Studies, 29.04.2021 23:50

Mathematics, 29.04.2021 23:50

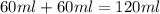
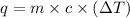
 = specific heat of water =
= specific heat of water = 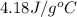
 = change in temperature =
= change in temperature = 
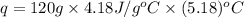
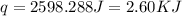
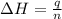
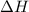 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?