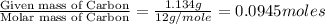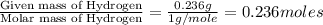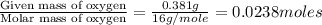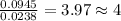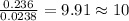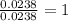Chemistry, 19.02.2020 00:00 anitadefrances
The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and oxygen. A 1.751 g sample of ether was combusted in an oxygen rich environment to produce 4.159 g of CO 2 ( g ) and 2.128 g of H 2 O ( g ) .
Insert subscripts to complete the empirical formula of ether.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1


Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and o...
Questions

Mathematics, 05.05.2021 20:40

Mathematics, 05.05.2021 20:40

Mathematics, 05.05.2021 20:40

Biology, 05.05.2021 20:40

Mathematics, 05.05.2021 20:40

Mathematics, 05.05.2021 20:40







Computers and Technology, 05.05.2021 20:40

Mathematics, 05.05.2021 20:40

Mathematics, 05.05.2021 20:40

Mathematics, 05.05.2021 20:40

Mathematics, 05.05.2021 20:40

Geography, 05.05.2021 20:40

Chemistry, 05.05.2021 20:40

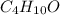
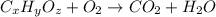
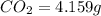
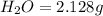
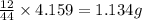 of carbon will be contained.
of carbon will be contained.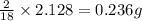 of hydrogen will be contained.
of hydrogen will be contained.