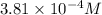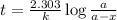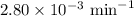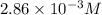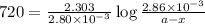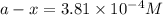G The gas phase decomposition of sulfuryl chloride at 600 K SO2Cl2(g) SO2(g) + Cl2(g) is first order in SO2Cl2 with a rate constant of 2.80×10-3 min-1. If the initial concentration of SO2Cl2 is 2.86×10-3 M, the concentration of SO2Cl2 will be M after 720 min have passed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

You know the right answer?
G The gas phase decomposition of sulfuryl chloride at 600 K SO2Cl2(g) SO2(g) + Cl2(g) is first order...
Questions

Mathematics, 22.11.2019 09:31

Mathematics, 22.11.2019 09:31



Physics, 22.11.2019 09:31

English, 22.11.2019 09:31



Mathematics, 22.11.2019 09:31


Mathematics, 22.11.2019 09:31


Mathematics, 22.11.2019 09:31

Mathematics, 22.11.2019 09:31

History, 22.11.2019 09:31

History, 22.11.2019 09:31


Mathematics, 22.11.2019 09:31

English, 22.11.2019 09:31

History, 22.11.2019 09:31