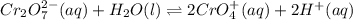Chemistry, 18.02.2020 21:48 Kennedy3449
Consider an acidic chromate-dichromate system at equilibrium in which the color is an orange-yellow. The reaction is represented by the equation:Cr2O72-(aq) + H2O(l)↔ CrO42+(aq) + H+(aq) orange yellow Which of the following best describes what happens if a strong base is added to the system at equilibrium? Explain your answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2


Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
Consider an acidic chromate-dichromate system at equilibrium in which the color is an orange-yellow....
Questions




History, 01.09.2019 10:20

English, 01.09.2019 10:20




Mathematics, 01.09.2019 10:20

English, 01.09.2019 10:20



English, 01.09.2019 10:20





History, 01.09.2019 10:20


English, 01.09.2019 10:20