
Chemistry, 18.02.2020 19:53 hannahking1869
The 116-g sample was heated to 94.5°C and placed into a calorimeter containing 72 g of water at 20.0°C. The heat capacity of the calorimeter was 14.7 J/K. The final temperature in the calorimeter was 25.6°C. What is the specific heat capacity (in J/g°C) of the mineral?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1


Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1
You know the right answer?
The 116-g sample was heated to 94.5°C and placed into a calorimeter containing 72 g of water at 20.0...
Questions



Computers and Technology, 22.08.2019 04:10


Computers and Technology, 22.08.2019 04:10






Computers and Technology, 22.08.2019 04:10



Law, 22.08.2019 04:10



Law, 22.08.2019 04:10

Computers and Technology, 22.08.2019 04:10


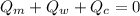
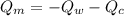 ----------------equation (1)
----------------equation (1)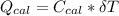 ---------------- equation (2)
---------------- equation (2)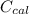 = heat capacity of the calorimeter
= heat capacity of the calorimeter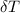 = change in temperature
= change in temperature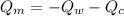
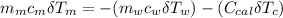
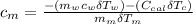 ---------------- equation (4)
---------------- equation (4)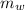 ) = 72g
) = 72g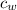 ) = 4.18 J/g.k
) = 4.18 J/g.k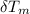 ) = 25.6 °C - 94.5 °C
) = 25.6 °C - 94.5 °C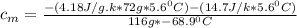
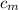 = 0.221172 J/g°C
= 0.221172 J/g°C

