
Chemistry, 18.02.2020 17:15 griffinadrianne946
Diamond and graphite are two crystalline forms of carbon. At 1 atm and 25∘C diamond changes to graphite so slowly that the enthalpy change of the process must be obtained indirectly. Determine △ Hrxn for C(diamond) → C(graphite) with equations from the following list: (1) C(dianond)+O2(g)⟶CO2(g)ΔH=−395.4kJ (2) 2CO2(g)⟶2CO(g)+O2(g)ΔH=566.0kJ, (3) C(graphite)+O2(g)→CO2(g)ΔH=−393.5kJ , (4) 2CO(g)⟶C(graphite)+CO2(g)ΔH=−172.5k J.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

You know the right answer?
Diamond and graphite are two crystalline forms of carbon. At 1 atm and 25∘C diamond changes to graph...
Questions




Mathematics, 29.10.2019 06:31

Chemistry, 29.10.2019 06:31

Biology, 29.10.2019 06:31







Mathematics, 29.10.2019 06:31


Mathematics, 29.10.2019 06:31



History, 29.10.2019 06:31

History, 29.10.2019 06:31

Mathematics, 29.10.2019 06:31

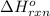 for the reaction is -1.9 kJ.
for the reaction is -1.9 kJ.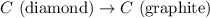
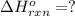
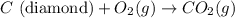
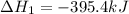
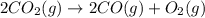
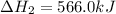 ( × 2)
( × 2)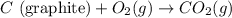
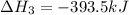
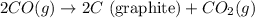
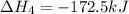 ( × 2)
( × 2)![\Delta H^o_{rxn}=[1\times (\Delta H_1)]+[2\times \Delta H_2]+[1\times (\Delta H_3)]+[2\times \Delta H_4]](/tpl/images/0514/1921/04537.png)
![\Delta H^o_{rxn}=[(1\times (-395.4))+(2\times (566.0))+(1\times (-393.5))+(2\times (-172.5))]=-1.9kJ](/tpl/images/0514/1921/ca977.png)



