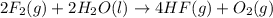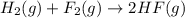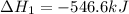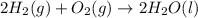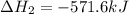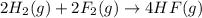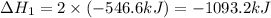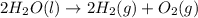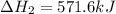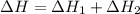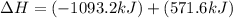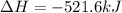Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
Given that H2 (g) + F2 (g) ⟶ 2HF (g) ΔH ∘ rxn = − 546.6 kJ 2H2 (g) + O2 (g) ⟶ 2H2O (l) ΔH∘rxn = − 57...
Questions

Geography, 24.07.2021 14:00



Mathematics, 24.07.2021 14:00


Social Studies, 24.07.2021 14:00


Mathematics, 24.07.2021 14:00


Biology, 24.07.2021 14:00

Mathematics, 24.07.2021 14:00




Mathematics, 24.07.2021 14:00

English, 24.07.2021 14:00

English, 24.07.2021 14:00

Chemistry, 24.07.2021 14:00


Biology, 24.07.2021 14:00

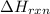 for the reaction is, -521.6 kJ
for the reaction is, -521.6 kJ