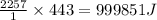Chemistry, 18.02.2020 06:27 rickyortega72701
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature. On average, an athlete loses approximately 443 g of sweat during an hour of exercise. How much energy is needed to evaporate the sweat that is produced? The heat of vaporization for water is 2257 J/g.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature...
Questions

Social Studies, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01








Mathematics, 28.08.2020 21:01



English, 28.08.2020 21:01


Chemistry, 28.08.2020 21:01

Social Studies, 28.08.2020 21:01


English, 28.08.2020 21:01


History, 28.08.2020 21:01