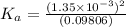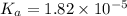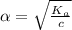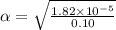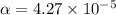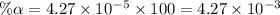Chemistry, 18.02.2020 05:22 Mangolinux7173
Start with 100.00 mL of 0.10 M acetic acid, CH3COOH. The solution has a pH of 2.87 at 25 oC. a) Calculate the Ka of acetic acid at 25 oC. b) Determine the percent dissociation for the solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
Start with 100.00 mL of 0.10 M acetic acid, CH3COOH. The solution has a pH of 2.87 at 25 oC. a) Calc...
Questions

Mathematics, 03.12.2020 06:40

Mathematics, 03.12.2020 06:40



English, 03.12.2020 06:40

Law, 03.12.2020 06:40

Biology, 03.12.2020 06:40


English, 03.12.2020 06:40

Mathematics, 03.12.2020 06:40


Mathematics, 03.12.2020 06:40


Physics, 03.12.2020 06:40



Mathematics, 03.12.2020 06:40

Social Studies, 03.12.2020 06:40

Mathematics, 03.12.2020 06:40

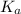 of acetic acid at
of acetic acid at 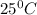 is
is 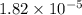
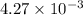
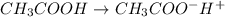
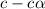
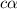
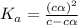
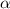 = ?
= ?![pH=-log[H^+]](/tpl/images/0513/9443/15713.png)
![2.87=-log[H^+]](/tpl/images/0513/9443/3a07c.png)
![[H^+]=1.35\times 10^{-3}M](/tpl/images/0513/9443/01bcb.png)
![[CH_3COO^-]=1.35\times 10^{-3}M](/tpl/images/0513/9443/0a4ad.png)
![[CH_3COOH]=(0.10M-1.35\times 10^{-3}=0.09806M](/tpl/images/0513/9443/5420f.png)