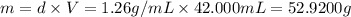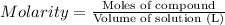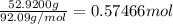Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1

You know the right answer?
Calculate the molarity of a solution of glycerol made by dissolving 42.000 mLmL glycerol at 15 ∘C∘C...
Questions







Computers and Technology, 22.11.2019 05:31








Mathematics, 22.11.2019 05:31