

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
Consider the following multistep reaction:
C+D⇌CD(fast)
CD+D→CD2(slow)
CD...
C+D⇌CD(fast)
CD+D→CD2(slow)
CD...
Questions

Mathematics, 13.03.2020 18:28





Chemistry, 13.03.2020 18:28





Law, 13.03.2020 18:28

Mathematics, 13.03.2020 18:28








![\text{Rate}=k'[C][D]^2](/tpl/images/0513/7830/4eb88.png)
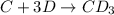
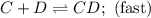
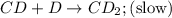
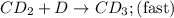
![\text{Rate}=k[CD][D]](/tpl/images/0513/7830/52b27.png) ......(1)
......(1)![K=\frac{[CD]}{[C][D]}](/tpl/images/0513/7830/c2f5d.png)
![[CD]=K[C][D]](/tpl/images/0513/7830/b2574.png)
![\text{Rate}=k.K[C][D]^2\\\\\text{Rate}=k'[C][D]^2](/tpl/images/0513/7830/6c083.png)


